Science
Related: About this forumSmooth trends in fermium charge radii and the impact of shell effects
Recently I've been writing down some ideas I've had for americium fueled nuclear reactors for the benefit of my son, so he has these ideas in writing in case during his life, after mine is done, he thinks them worthy of attention.
The use of americium fuels will inevitably result in the production of higher actinides, chiefly isotopes of curium and californium, both of which have interesting properties suitable for use, but also, particularly under "breed and burn" scenarios to which americium fuel is particularly well suited, as the fission of americium isotopes results in high neutron multiplicity, fairly large quantities, perhaps grams and even kilograms of curium and californium's higher isotopes. Under these conditions, involving potentially years or decades of continuous operation without refueling, neutron capture reactions in californium isotopes should result in small quantities of einsteinium, in particular, some of the relatively long lived isotope 254Es (t1/2 = 275.7 days). The quantities obtained would be very small, since many of the precursors would be subject to nuclear fission in a neutron flux, with only some of the isotopes capturing neutrons without undergoing fission. The diminishment of the actinide targets is shown in the following diagram.
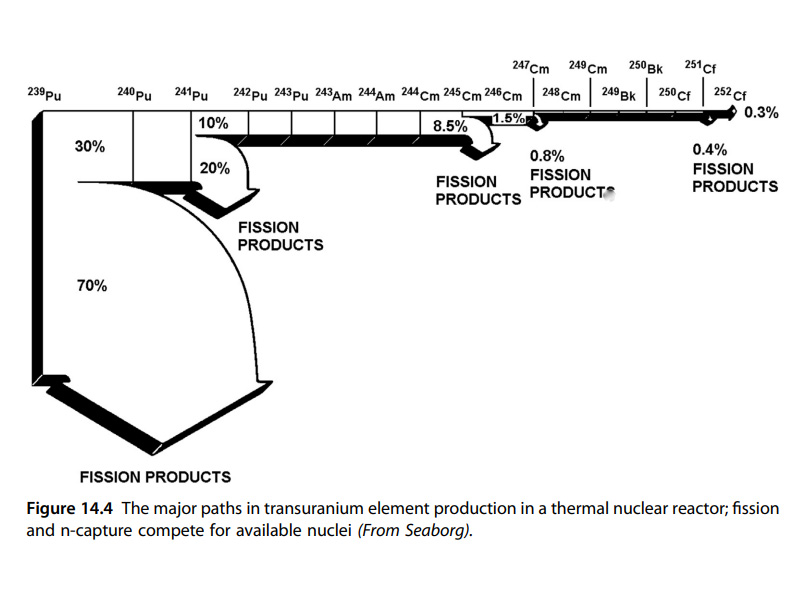
cf: Gregory Choppin, Jan-Olov Liljenzin, Jan Rydberg, Christian Ekberg, Chapter 14 - The Actinide and Transactinide Element, pages 405-444 (2013) figure 14.4
Einsteinium and Fermium were first identified as residue of the first thermonuclear weapons test, the "Mike" test in 1952. I have rather long wondered how this was accomplished, since obviously, it would involve traveling, a relatively short period after the detonation of this massive weapon, into the highly radioactive zone shortly after the detonation of the device.
A rather detailed, description of how this was accomplished is provided in this very interesting document including the major participants in the discovery in a 1978 symposium which is available on the internet:
The 1978 Symposium on the Discovery of Einsteinium and Fermium
It involved on one hand, fighter jets flying through the mushroom cloud with filter papers attached to their wings, and the collection, sometime afterwards, of about 500 kg radioactive coral to the United States for reprocessing and reduction to atomic scale examples of the two elements, using detectors developed by Mr. Albert Ghiorso, who is listed as co-discoverer, along with Nobel Laureate Glenn Seaborg, of 11 elements in the periodic table, but (I didn't know this) seems to have never been awarded a Ph.D.
Ghiorso was given the honor of suggesting names for the new elements, and he chose Einsteinium and Fermium in honor of those among the greatest minds of the 20th century, somewhat ironic in the sense that Albert Einstein pointedly opposed the development of thermonuclear weapons, a point he made when interviewed by Eleanor Roosevelt in her radio show in 1950.
It is interesting that, despite handling some highly radioactive debris, almost all of the participants in this adventure were still alive in 1978, twenty five years later, with the exception of Glenn Seaborg's high school friend and later scientific collaborator, Stan Thompson, who designed the first industrial process for isolation of plutonium, the bismuth phosphate process. I actually attended a lecture in 1994, in San Diego at an ACS meeting, where the subject was - I kid you not, Einsteinium - when Glenn Seaborg sat in the chair next to me, whereupon I was entirely too awestruck to say a word. (Seaborg died in 1999, at the age of 87.)
These details of this are available at the link to the 1978 symposium.
The discovery was a surprise to the participants, because it suggested that in a very high neutron flux, approximately 1024 n/cm2, about a trillion times higher than is observed in a commercial nuclear reactor, 238U can capture in minute fractions of a second, 15 or more neutrons, which rapidly decay to the higher actinides up to and including fermium (and possibly beyond). This is probably the state of affairs observed in stellar explosions such as supernovae which account for the natural radioactivity of the Earth.
Another account of the discovery of these elements, far more abbreviated is here:
Becker, S. A. (2023). The Serendipitous Discovery of the New Elements Einsteinium and Fermium from the Debris of the Mike Thermonuclear Test. Fusion Science and Technology, 80(sup1), S105–S109
In this recent paper, a discussion of the process in the Mike test is compared to the stellar "r-process" which accounts for the creation of heavy elements, including the large quantities of uranium and thorium (and their decay daughters) now present on Earth.
In the next step (the prompt process), the target seed nuclei (typically starting with 238U, although other isotopes like 232Th, 242Pu, and 243Am have also been used) undergo a series of (n, gamma)reactions (up to 17 in the case of the Mike test). The neutron-capture phase is so short that there is no time for any Beta- decays of the neutron-rich isotopes. Competing with the (n, gamma)reaction are neutron-induced fission and the (γ,n) reactions, which work to reduce the net abundance of the very neutron-rich isotopes. The final step is called the “decay back process,” during which a neutron-rich isotope like 255U undergoes a series of eight Beta- decays to the line of Beta- stability to become 255Fm. The final isotopic results of the decay back process can also be affected by spontaneous fission and Beta- delayed fission...[Citation10]
The prompt process is somewhat similar in nature to the astrophysical r-process, and the two processes are compared in Table II.[Citation11] The r-process is currently thought to take place in supernova explosions of massive stars and as a result of neutron star–neutron star collisions. Both the prompt process and the r-process result in rapid, multiple neutron captures on seed nuclei that produce nuclei far from the line of Beta- stability. As previously discussed, the prompt process takes place so quickly that there is no time for Beta- decays until after the neutron-capture phase has ended; by comparison, the timescale for the r-process allows some Beta- decays to occur during the neutron-capture phase.
The higher-temperature environment for the r-process also means that the neutron-capture cross sections for the seed nuclei would be smaller than they would be under the prompt process. The similarities between the r-process and the prompt process are, however, such that the data from a number of nuclear tests have been used to calibrate the nuclear physics used in astrophysical r-process nucleosynthesis codes...
(To address the limitations of the DU editor which does not allow greek letters, I have replaced the greek letters with their spelled names.)
Einsteinium is the element with the highest atomic number that has been isolated in visible quantities. It has been reduced to the metallic form, on a milligram scale. Its high radiation has made determination of its physical properties, such as its crystal structure - thought to differ from other actinides - problematic.
It has a power output of over 1000 Watts per gram; a full gram has never been sythesized.
An interesting paper on its nuclear chemistry, along with photographs of the actual processes involved in isolation of these elements is available in an open source format:
Roberto, J.B., Du, M., Ezold, J.G. et al. Actinide targets for the synthesis of superheavy nuclei. Eur. Phys. J. A 59, 304 (2023).
The preparation of visible amounts of the element has allowed for an understanding of its chemistry and certain properties associated with its nuclear physics.
The article, from which the title of this post is taken is here:
Warbinek, J., Rickert, E., Raeder, S. et al. Smooth trends in fermium charge radii and the impact of shell effects. Nature 634, 1075–1079 (2024)
The article is open sourced, and interested parties can read it for free.
An excerpt from the introduction, including reference to the very stable isotope of lead, 208Pb is provided for convenience:
Nuclei with proton numbers residing between magic numbers are expected to have deformed shapes owing to the nuclear Jahn–Teller effect8,9. The stabilization of deformed nuclei can be associated with the reduced density of the deformed single-particle levels of the nuclear mean field6. In the region of heavy nuclei beyond 208Pb, a deformed subshell at N =152 was early identified through irregularities in the systematics of the alpha-decay energies of californium (Z = 98) isotopes, deviating from spherical shell model considerations10. Recently, precise mass measurements enabled a direct investigation of the N = 152 neutron shell gap in nobelium (No, Z = 102) and lawrencium (Lr, Z = 103) isotopes. The size of this subshell was determined from the experimental binding energies to be about a factor of four weaker than in 208Pb (refs. 11,12,13). As illustrated in Fig. 1 (top), the N =152 gap gradually decreases in the lighter isotones. This result, consistent with spectroscopic studies14 and the recent analysis of experimental and theoretical binding energies15, confirms the local nature of this shell effect...
Further on...
The isotopes studied on-line were available through the following schemes: direct production of 245,246Fm in fusion-evaporation reactions, indirect production of 248,249,250,254via the decay of directly produced 252,253,254No, and indirect production of 255No via the electron-capture decay branch of 255Lr. These indirect production schemes evolved from recent methodical advancements that gave access to previously inaccessible isotopes34 (for details, see Methods). However, the isotopes 251−253Fm are currently not accessible by this technique, not least owing to long half-lives of more than 5 hours up to several days...
Interesting stuff, I think.
erronis
(20,422 posts)In my short life-time I've watched the periodic table expand and get filled in with actual sightings of some of these very rare elements. I actually met Glenn Seaborg in the early 60s in Geneva at the UN. Unfortunately I was too young to understand his stature.
Do you think there may be significant discoveries yet to be made in these heavier transuranic elements? Islands of relative stability from radioactive decay? I think I've read some new research indicating that certain isotopes of some non-naturally occurring elements may have increased stability. And what would be the implications?