Environment & Energy
Related: About this forumNovel Extractants for the Separation of Americium from Lanthanides in Used Nuclear Fuel.
The paper I'll briefly discuss in this post is this one: Am(III)/Eu(III) Extraction and Separation with N-heterocyclic-Derived Unsymmetric Amide-triazine Extractants Xiaofan Yang, Linjia Chen, Dong Fang, Yaoyang Liu, Shihui Wang, Lei Xu, Anyun Zhang, Jing Su, Chao Xu, and Chengliang Xiao Industrial & Engineering Chemistry Research 2025 64 (12), 6692-6703.
I've been thinking quite a bit about americium of late, since I regard it as a key element for sustaining nuclear energy - the last, best hope of humanity - while minimizing, even eliminating, the need for mining perhaps for generations.
A recent post of mine on the topic, given americium's potential to recover the neutrons that went into its generation, is this one: Some Aspects of the Use of Americium as a Nuclear Fuel
I'm not a solvent extraction kind of guy. I prefer fluoride volatility separations because they can isolate neat uranium, neptunium, and plutonium fluorides in the +6 oxidation state, and because many of the fission product metals, among them rhodium, ruthenium, molybdenum and technetium, also exhibit volatile fluorides that can be separated by fractional distillation.
However what is true of the widely used PUREX solvent extraction procedure in terms of residues is also true of fluoride volatility. In particular, the residues of fluoride volatility will contain a mixture of lanthanide elements, lanthanum through gadolinium, mixed with the higher actinides americium, curium, traces of berkelium and californium, all of which are valuable. The lanthanides do not form volatile fluorides, nor do the higher actinides; all efforts to make americium hexafluoride have failed. The fluoride volatility separation will also include residues of cesium and rubidium fluoride - which can be washed out with water - the former will be highly radioactive and thus valuable, and insoluble barium and strontium fluoride, and some transition metals that do not form volatile fluorides, notably palladium, cadmium and silver.
Nevertheless, fluoride volatility methods will remove and separate better than 96% of the mass of used nuclear fuel as a minimum, making solvent extraction of the residues less chemically intensive.
From the paper's introduction:
Due to their identical charge (+3) and comparable ionic radii (rLn(III) = 120.6–102.4 pm, rAm(III)–Lr(III) = 115.7–107.4 pm, CN = 9), An(III) and Ln(III) exhibit strikingly similar chemical properties. (6,7) Conventional extractants, such as di(2-ethylhexyl)phosphoric acid (HDEHP) and tri-n-octylphosphine oxide (TRPO), show similar extraction efficiencies for both An(III) and Ln(III), as both belong to the f-block of the periodic table. (8−10) The challenge of developing extractants with enhanced selectivity for An(III) over Ln(III) lies in identifying subtle differences in their chemical properties, a long-standing issue in the fields of nuclear chemistry and radiochemistry. (11,12) According to the Hard and Soft Acids and Bases (HSAB) theory, An(III) is a softer acid due to its more diffuse 5f electron cloud and tends to form stronger complexes with softer donor atoms, such as nitrogen (N) and sulfur (S). (13−15) Extractants incorporating multiple donor atoms and forming appropriately sized coordination cavities can enhance the interaction between the extractant and the target ion. (16,17) Based on these principles, multidentate chelating agents incorporating nitrogen-containing heterocyclic skeletons have been designed and applied to the extraction and separation of An(III) and Ln(III). (18−22)
I emphatically agree with the first 13 words of this excerpt, and have no problem with the rest of it either. Humanity will suffer greatly, more than it already is doing, without access to nuclear energy, which to my mind is the only acceptable form of primary energy, although - a minor quibble with the text - "acceptance" is not as wide spread as it needs to be, since obviously in these times, ignorance remains more powerful than ever.
Here are the structures of some of the extractants:
The caption:
The paper focuses on entries (e) and (f) which are renamed L1 and L2
The following figure shows the distribution coefficients under various conditions of acidity and concentration:
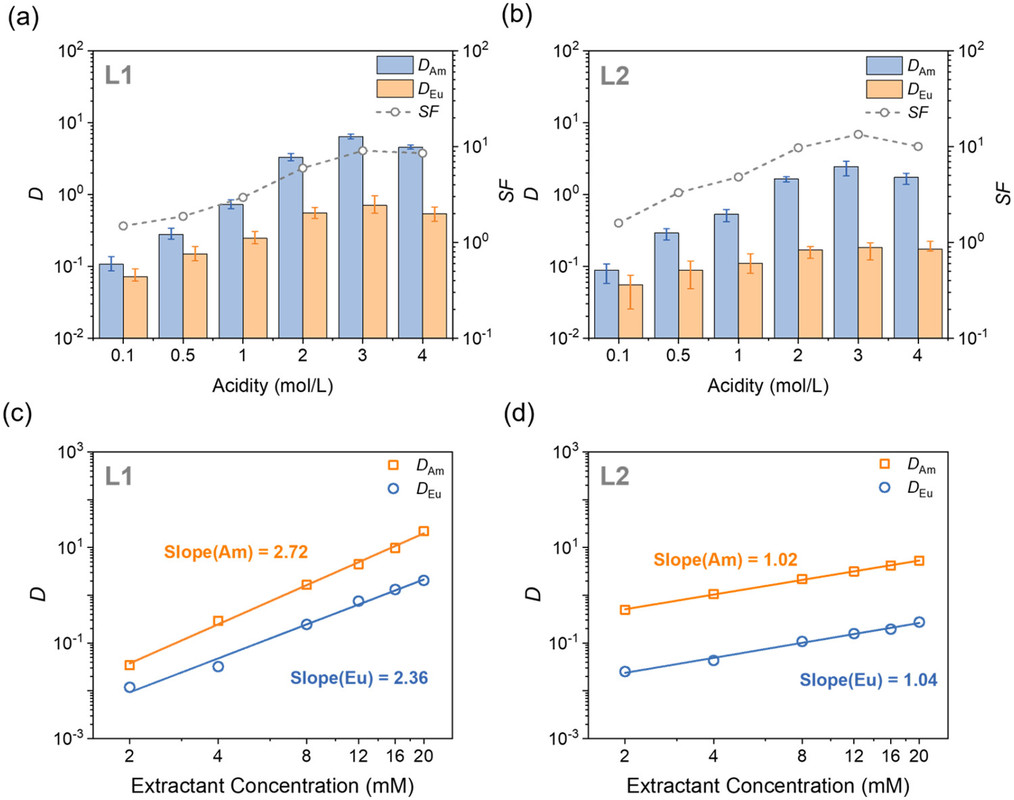
The caption:
From the paper's conclusion:
From my perspective, this technology might be improved with some changes, which may or may not include metathesis of fluoride anions for nitrate anions, but this is really not for me to say; I'm purely armchair.
It is however clear to me that we must recover and utilize all of the actinides if we are to have a sustainable world, although a sustainable world, from my perspective, seems less and less likely precisely because things like this are not taken seriously, and some of what is taken seriously is either horrible or absurd, often both.
Have a nice day tomorrow.